-
Posts
176 -
Joined
-
Last visited
Profile Information
-
Gender
Male
-
Location:
Montana
-
Interests:
Coinshooting, jewelry hunting, prospecting
-
Gear In Use:
Manticore, Equinox 700, Vista X, Tarsacci, Excalibur II, SeaHunter Mark II, DetectorPro UW, GPX-5000
Recent Profile Visitors
The recent visitors block is disabled and is not being shown to other users.
BigSkyGuy's Achievements

Copper Contributor (3/6)
349
Reputation
-

Any Peeps On Here Who Regret Purchasing The Mighty Manticore?
BigSkyGuy replied to Guinea1's topic in Minelab Manticore Forum
No regrets here! I have found silver in parks which have been pounded by the 800 and other machines. One thing that I noticed was, when I first got the Equinox I got signals that were obvious "dig me" signals that turned out to be silver. On the Manticore the remaining signals have been more iffy but I was still able to squeeze out a few more finds from the same places. Yes, I have dug a bit more iron, but it has been worth it for me. I just tried the M8 a couple of days ago and was generally pleased. Nice and light, great separation. As others have mentioned, it does up-average more than the M11. I got a solid signal in the 90s that turned out to be a wheat at 6 inches. As long as you recognize this, I guess it does not matter too much. The target trace is useful, but I must admit that I do not feel like I have mastered it yet. More information is always better in my book. As far a the Deus 2, I have not tried it, but the stuck pig squeals that come out of the XP machines have never appealed to me. Just my opinion of course. -
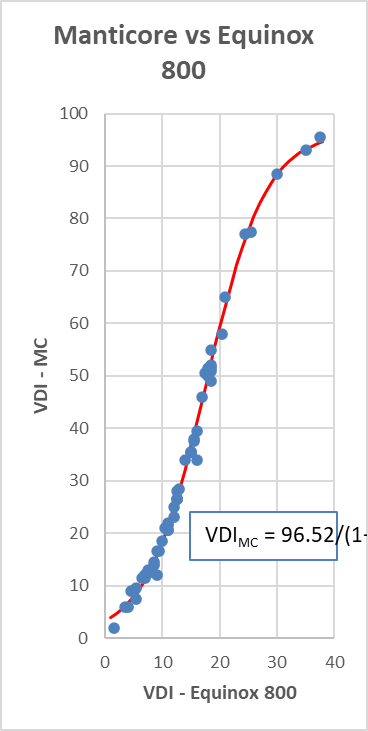
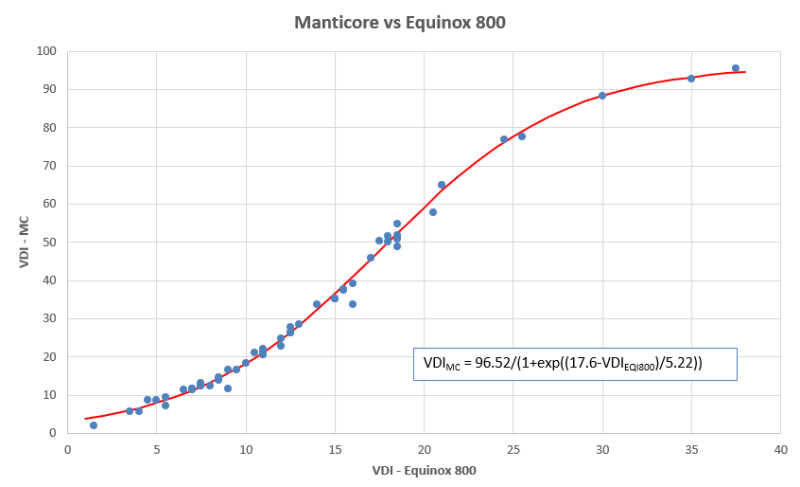
Strick, My interpretation of the graph is that the numbers were expanded in the middle of the range, but not so much on the high and low ends. It you approximate a straight line through the highest three points, the slope looks to be less than 1, meaning that the Equinox actually has slightly more resolution in this area (i.e. more numbers between a US quarter and a silver dollar). Near the middle of the curve, the slope is much higher than 1, indicating that the MC has an expanded range in this area. I think in this area you are correct, that the numbers are approximately doubled (e.g. for a nickel this is true, 12-13 for EQI vs 26-27 for the MC). I would guess that this was intentional. There is no real benefit from expanding the range between a quarter and a dollar, but some possible benefit in the middle of the range where pull tabs and gold jewelry are present (but this is debatable of course).
-

AlgoForce E1500 Vs The Rest
BigSkyGuy replied to Steve Herschbach's topic in Detector Prospector Forum
Exactly why I am interested. I have a 5000 with lots of coils and no desire to drop several thousand for a ML PI gold detector.- 45 replies
-
- 6
-

-
- minelab gpx
- algoforce
-
(and 1 more)
Tagged with:
-
The low one was due to too much manganese being added at the mint.
-
Based on my tests it appears to be a leaching issue. I tested over 2,000 non-dug war nickels and only found three out of spec. @JCR has found several War Nickels that read high, but I suspect that he has not recovered thousands of them that read in spec. Also of interest, is that one of the three out of spec coins that I identified read low. I have not heard of anyone finding one the reads low. As GB mentioned the manganese was added to decrease the overall conductivity of the coin so that it matched the standard 75% copper/25% nickel coin. Under mildly oxidizing conditions, manganese metal oxidizes to a very soluble ion (Mn+2), unlike copper which forms a relatively insoluble oxide coating on the metal. Therefore, the manganese leaches from the coin, increasing the conductivity.
-
Thanks GB. The winters are long and cold in Montana, so I have to keep myself busy!
-
Last winter I had the opportunity to test 2,008 non-dug War Nickels that I borrowed from a coin dealer friend. Out of 2008, 3 were out of spec (0.15%), which he let me keep. Two biased high and one low as shown in the pic. The numbers refer to VDI on the Manticore, AT-G mode. The mint was very careful to match the weight and conductivity of the standard 75% copper/25% nickel coin so that they could be used in vending machines. Having a rate of 0.15% out of spec coins seems very good, given the difficulty in alloying manganese with the other metals. The rate of dug nickels which read anomolously high is well above the 0.15% rate coming from the mint, so the manganese leaching theory seems plausible.
-
Counterfeit 2 reales were very common during Colonial times. In fact several articles have been written on the subject. Two from Circulating Counterfeits of the Americas EDITED BY John M. Kleeberg in the Coinage of the Americas Conference at the American Numismatic Society, New York November 7, 1998 are: JOHN M. KLEEBERG. Counterfeit 2 Reales of the Bust Type: Charles III, Charles IV, Ferdinand VII, 1771-1821 A Survey and Die Study 137 JOHN P. LORENZO The Counterfeit Spanish Two Reales: Canadian Blacksmiths or North American Tokens 193 These are available online at Archive.org
-

Some Things To Take Note Of With The Algoforce
BigSkyGuy replied to phrunt's topic in AlgoForce Metal Detectors
Phrunt, Does this mean that you do not see the bars increase for non-ferrous targets? Less increase for non-ferrous? Thank you! -
Just curious if anyone has purchased anything from the DetechDetect.com website and if so, what your experience has been? Are there any issues with customs fees coming into the US? Some of the prices on Detech coils are quite low (i.e. 199 Euros for a 15" DD Spiral, which works out to about $214 USD). The company associated with this website is Asheras Ltd in Silistra Bulgaria. Perhaps they are a Detech distributor which allows them to sell at lower prices? The Detech USA website has been down since the outbreak of COVID. Thank you!
-

Forget Algoforce, You Need This!
BigSkyGuy replied to Steve Herschbach's topic in Detector Prospector Forum
Gerry is right. Here is a KellyCo ad from Treasure Magazine October 1978. The Dar Nel rod looks like it was fashioned from a coat hangar. Not nearly as fancy as the one that Steve posted with four antennas! One of them looks like a Bic pen.