-
Posts
5,808 -
Joined
-
Last visited
Content Type
Forums
Detector Prospector Home
Detector Database
Downloads
Everything posted by GB_Amateur
-
Welcome, AdamsDetecting! Can you tell us more about your detecting plans and experience?
-
Why is P.T. Barnum whispering a counterargument in my ear? Or is it Mark Twain? To name just two of many homespun armchair philosophers. No question that ML has some really smart people and have made some equally intelligent decisions. But that never covers everything that occurs or doesn't occur. (Given your criticism of the robustness of the Equinox, I'm confident you agree with that statement.)
-
Although I didn't have a 1909-S to measure (no surprise there since it's a semi-key), you can see in the above table (in Part 4 -- Summary section) that even from 1916-1925 the dTID's for undug -S mintmarked Lincolns is around 20, consistent with pre-1894 IHP's. So maybe this is further evidence that higher tin content (compared to most Lincolns, especially post WWII issues) leads to more attractive green toning without scaling. Again, that is my hypothesis that the dTID for undug coins correlates inversely as the tin concentration. For dug coins it seems (with limited data) that there is a (further) small reduction in dTID that appears to be independent of tin content. Thanks for your expression of appreciation -- always welcome and especially from a highly experienced detectorist.
-
I don't have enough 90% silver USA coins to do such a detailed study. There may be reports here of variations of dTID's among those but I don't recall (warning: aging memory) any at this time. (The WWII 5 cent coins with 35% silver are an exception but IMO the most likely explanation is a ground effect rather than a mint composition variation. That has been discussed in a thread a couple years ago. My measurements of 160 undug coins showed no appreciable dTID variation. That doesn't rule out a mint composition anomaly in rare instances, though.) I realize you wrote this prior to my posting of part 3. My dug IHP's and many Lincolns (both Wheats and 95% copper Memorials) both show a green color. However, in general the scaling seems to be much worse on the Lincolns. I don't know if that is due to a higher percentage of tin (still supposedly capped at 5%) for the IHP's or not, but that is my initial suspicion. Some of the IHP's (as has been shown in photos here by other detectorists) can actually be attractive with their green cast. That doesn't seem to ever happen for the green-scaly Lincolns I've dug. I appreciate all of the (few, at this writing) likes and comments regarding my report. For those who have chosen (for whatever reason) to ignore it, I have no problem with that other than I sure hope it's not contradicted in the future by lack-of-evidence posts as has been the (admittedly rare) case previously. There is always room for disagreement but if information is not evidence based, it ought to be labeled and viewed as such. At least that's my stance and I will continue to question, as has been my practice, rather than to ignore and/or sweep such posts 'under the rug.'
-
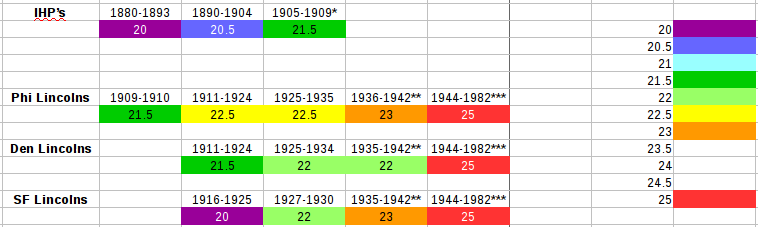
Part 3. Effects of soil on the dTID of metal detecting found USA small cents. Parts 1 and 2 only show dTID measurements for coins that have never been (at least to my knowledge) in the ground. Although those were exposed to chemicals (e.g. skin oils, typical air molecules) I found no indication when comparing uncirculated (think 'untouched by human hands') coins and those that obviously had been in common day-to-day usage. Even wear (loss of detail and mass) did not show a dTID change. Cautionary Note: Coins recovered from the ground introduce multiple new variables. Most of my dug coins are from a small geographic area with more/less consistent soil composition and typical moisture levels. Those characteristics are going to be different for other parts of the USA, so what I show below may or may not be even relevent let alone representative of what other detectorists experience. The length of time spent in the ground quite likely affects the dTID change. (There are some indications in my data that this is the case but it is far from universal.) Further, knowing how long a coin was in the ground is generally uncertain. Finally, even in consistent soil, local differences such as types of vegetation (especially tree varieties) likely play a role. My plots below result from subtracting the dTID of undug coins from the same date+mintmark of dug coins. For example, if my average value for undug coins (as shown previously) for a particular date+mintmark was 25 and a dug coin of the same date+mm had a dTID of 24, the datapoint below will show the difference: 24 - 25 = -1. (Note: To illustrate the 0 valued coins I put in a small negative value. Also, the one positive value in the Lincoln plot is likely due to measurement error as opposed to a real effect.) These two plots should not be compared as apples to apples. The IHP data are for all 14 Indian Heads I've ever dug, regardless of condition. For the Lincolns, I intentionally selected the nastiest green scaled coins for which I could see date+mm. Thus although the largest changes were among the Lincolns, it would be a mistake to conclude that Lincolns are more susceptible to dTID changes than IHP's. Another selection effect is that I typically have used a dTID of 20 when searching to mentally discriminate and make a dig/no dig decision. I.e. at least part of the signal when sweeping over a target had to be 20 or above (or 12-13 for USA 5 cent 'nickels'). If an IHP in the ground had a lower dTID (e.g. 18-19) I would have skipped over it. This biases against the largest dTID differences for the IHP's. The Lincoln plot includes both Wheat and Memorial (95% copper composition) cents. I mentioned a possible correlation with time in the ground. Worth mentioning(?) is that the leftmost two datapoints (both dTID differences of -2) were the two oldest green-scaly Lincolns of the 25 measured -- 1916 and 1920 (both without mintmarks meaning minted in Philadelphia). However, the more recent Memorial cents (1959 and later) are located all over this plot, on the left and the right. As far as exactly why the ground degrades the dTID, it's not obvious to me. @PimentoUK has mentioned leaching (selective removal of a particular alloy component) as playing a role. Is this only a surface effect or a bulk effect? If only a surface effect, does that impact the skin depth, resulting in a lower dTID response? That's only two possible causes out of potentially many. Regardless, the evidence above supports a change (lowering) of dTID for (some) 95% copper USA small cents that have spent considerable time in the ground. Part 4. Summary. I've presented quite a bit of information in this series of posts. As such a (short! 😁) summary of my findings seems in order. 1) USA small cents that have never been in the ground exhibit different dTID's (specifically on the Minelab Equinox but quite likely on other detectors as well), particularly dependent upon date but also the mint where produced even for the same date. 2) Some of those variations are consistent with known composition variations specified by mint officials, but others seem to defy those specifications and still other known composition differences appear to lead to non-existant dTID differences, possibly due to the changes in composition being quite small. I reiterate that since I've never seen specifications of allowed tolerances in composition by (private) suppliers, it could be that some of those dTID differences are simply a reflection of allowed tolerance variations. 3) Even though the amount of tin is specified to be no more than 5%, the amount of zinc no more than 5%, and the sum of those two exactly 5%, it appears that an increased amount of tin (and thus a decrease amount of zinc) over this seemingly tight range is responsible for the lower dTID's measured. 4) Coins that have been in the ground, regardless of their composition when minted, can show a lowering of dTID. 5) Table showing general trend of dTID's over time (only coins not taken from the ground represented here): Note: This table is a generalization. There are exceptions, some of which were pointed out in parts 1 and 2. Also, there were Lincolns with San Francisco (-S) mintmarks during the 1909-1915 years but since I measured none of those there is a gap in the above table (prior to 1916). The Denver mint started producing cents (-D mintmarked) in 1911. * 1908-S (only one of two SF minted dates for the IHP's and thenot only one I have access to) had a dTID of 20. **1942 year had both pre-war (23) and intra-war (25) dTID's for my Philadelphia samples. As shown in part 2, my limited samples from the other 2 mints show only one or the other, but that doesn't rule out both compositions being produced at each of those branch mints. ***1982 was the transition year during which both {95% Cu, 5% Zn} and {2.5% Cu, 97.5% Zn} coins were minted. The 25 value is for the 95% copper version only. See parts 1 and 2 of this report for more details. Postscript: Since I had coins out and sorted I decided to look at one other question: how consistent are the weights of coins? I'm only measuring a small and very specific sample: 1982 95% copper cents from both Philadelphia and Denver mints (unseparated). I pulled these coins from circulation between 1982 and 1989. Over 95% show mint lustre, and in many cases barely show any browning from being in ciruclation at all. Thus these weights can be assumed to be as released, i.e. not exhibiting measurable wear. I think the mint's specificaiton is 3.11 g (or possibly 48 grains = 3.111 g to an extra decimal place). As you can see, the average comes out to be ~0.01 g below the specification, but again I don't know the tolerance. (Note: I calibrated the scale -- which reads to 2 decimial places for grams -- with 3.10 grams of calibration weights and it read spot on.) Finally I wanted to get a measurement of the effect of wear due to a coin being in circulation a long time. I chose six Lincolns dated 1911 which to eye were graded either About Good (AG-3) or Good (G-4). Their average weight was 3.03 g. Thus assuming 3.11 g initial (as minted) weight, the loss is only ~0.08 g or a modest 2.5%. (I measured the weight loss of a well worn Barber dime previously and posted the result on this website. I can't remember the fraction loss for sure but I think it was between 10% and 15%. That dime had an easily readable date so possibly an AG grade as well.)
-
This can also apply to the 'high-grader', i.e. if you are out prospecting, thinking you are on unclaimed land and are approached by the supposed claim owner. Sure, a couple percent of people are pure a__holes but most have some tolerance and even compassion. The 'non-confrontational' mode mentioned really can lead to positive solutions, or at worst, neutral solutions. Although I haven't experienced this in regards to mining claims, it has happened when detecting on what I thought was public, accessible property. Good stuff, Steven. Thanks for that post.
-
One thing's for sure, in my dry land sites anyway, the plastic liners don't deteriorate. If I find a rusty crown cap with no liner I assume it's because the cork deteorated away, although given how many aluminum pieces that were meant NOT to be broken off or removed are laying around loose I wouldn't put it past the idiots to remove the plastic liners from the caps and toss them, too.
-
That sucks. You mean you can't plug wired headphones into the control module? Do the onboard transmitters (Bluetooth and proprietary) still work? Do you use this detector in the water? (Sounds like if 'yes' then you'll have to add 'not anymore!') Sounds like for that price you'd be more than 1/2 way to a (3 year warrantied) N/M Legend and more than 1/4 of the way to a new XP Deus 2 (w/ 5 year warranty). If you can sell the Equinox coils, WM08, ML80's, etc. you're even closer to one of those brand spankin' new detectors. And maybe someone would buy your control module for parts. If ML won't repair control modules there are others out there with similar issues.
-
According to Wikipedia: Bobby pins became popular in the 1920s to hold the new bobbed hairstyles. Do they rust away? It seems in my parks I very rarely dig crown caps with cork liners, and when I do the metal is crumbling. I figure these earlier ones (maybe a different alloy than today) only have 50-60 year lifespan before they revert back to nature. It's also possible that their deterioration causes them to only ID in the iron range, which I almost never dig in any site (gold prospecting being the exception). Do you salt water detectorists (and salt water beach detectorists) find cork-lined crown caps?
-

Preventive Reinforcing Of Coil Ears
GB_Amateur replied to mcjtom's topic in Metal Detector Advice & Comparisons
For which detector? The answer to "does it make sense?" possibly depends upon the detector. JB Weld is a brand name and they have many products. Which one are you referring to? If you cure a bead's worth of it, say the size of a small coin, does your detector sound off on it? -
Isn't native silver pretty unusual? Could that specimen have collector value (well above it's bullion content)?
-
Part 2. Lincoln Cents 1909-1982. 2a. dTID's of coins from the Philadelphia Mint. (Note: Part 1 contains the experimental setup and details of the measurements. Those apply to this Part 2 as well.) In Part 1 of this study I showed the results of dTID measurements of Indian Head Cents (IHP's) with some inclusion of the early years of the Lincoln series for comparison. The plots in this Part 2 only show the last two years of IHP's, again for comparison purposes. Here is the next plot's worth of data: In that earlier section I noted the discontinuity in the Lincolns between 1910 and 1911. I speculated that although the Mint was given orders to use a composition of 95% copper, 3% tin, 2% zinc starting with the beginning of Lincoln Cent production, the data seem to show otherwise. I've found no evidence in the literature (see Part 1 for my references) that a change in composition specification was made until WWII. Yet the first two years of Lincoln data show consistency with the late IHP's followed by a ~32 year period of more/less consistent, higher value. There may be better quality control in the last few years of that time window (1936-42) but all measurements made for Philadelphia minted coins from that period are within the [21.5,23.5] interval (inclusive), and over 80% read either 22.5 or 23. Interestingly although it's seldom mentioned, according to the Bowers book (see Part 1), 1942 mintage contained a mix of pre-war and intra-war compositions (which I'll define shortly). My data confirm that, since the four 1942-plain (Philadelphia) coins read 22, 22.5, 24, 24.5. For the 1943 year the composition was changed drastically, reportedly so the metals in cents up to that time could be used for the war effort. The zinc coated steel (whose dTID I measured at negative 6.5 for two Philly specimens) proved unpopular and from 1944 through 1946 (also in practice during part of 1942) the composition was designated 95% copper, 5% zinc (thus no tin). This change is apparent in the data above. (As a sidelight, the copper used in those years was supposedly recycled from gun cartridge shell cases.) In 1947 it was ordered that the composition be changed to 95% copper, 1% tin, 4% zinc. There was no announced subsequent change (that I've been able to find) until 1962. Yet, surprisingly (to me, anyway), the dTID measurements don't show an obvious difference for this intra-war/post-war composition change, except for inconsistent (typically lower) values in the late 40's and first half of the 50's. (As you'll see below, this didn't happen for coins minted in Denver.) As was speculated for the early Lincolns (in Part 1), was this possibly due to using up old stock, in this case pre-war surplus sheets? Other possible explanations are failure of suppliers to meet requirements (which the mint let slip through) and even some measurement errors by me. (Note: I only measured one 1954 and one 1955 from Philly. I could do more....) Starting in 1962, tin was once again left out and the composition matched the war years (95% copper, 5% zinc) until sometime in 1982 when the composition was drastically altered, removing most of the copper and resulting in the coin detectorists' unpopular 'Zincolns'. The above plot (as well as those below for the branch mints) shows no change in dTID resulting from the removal of tin in 1962. Of course the removal of most of the copper in 1982 onward (becoming 97.5% zinc, 2.5% copper, the latter being partly in the core alloy and partly in a pure outer coating) is apparent in the dTID changing from ~25 to ~21. 2b. dTID's of coins from the Denver and San Francisco Mints. The last two plots show data measurements of -D and -S mintmarked coins from the same time period as above: Neither plot's dTID's line up well the previous Philadelphia data until after WWII. (This was the biggest surprise for me.) In the case of Denver minted cents (first minted in 1911), the dTID's through 1924 seem in line with the late IHP's and first two years of the Philly Lincolns. Even from 1925 to 1942 they are lower than Philly, roughly around 22 compared to Philly's ~23. But then from 1944 through the end of 95% copper mintage in 1982, the numbers appear consistent in dTID value but considerably more consistent than those of Philly minted coins! Meanwhile, San Francisco minted cents are different than those of either of its sister mints. (Note: I have no early teens from SF to measure.) From the late teens into the mid-20's the dTID's are consistent with the (Philly minted) IHP's of the 1880's! The late 20's coins are in line with Denver's issues from that time period while the late 30's are closer to coinciding with Philadelphia. Post war production in SF seems close to both other mints in dTID values but possibly shows inconsistencies similar to Philadelphia. In the late 60's and early 70's, dTID's of coins from all three mints finally appear to be consistent. (Note: For those not familiar with the Lincoln series, there were minor and major gaps in production of cents in San Francisco. For example, 1932-34 saw only Philadelphia and Denver issues, and no cents were minted in SF from 1956 through 1967. Since 1975 only collector 'proof' coins not intended for circulation have been made there. While we're on exceptions, an upheaval in USA numismatics was brought on by Congress to punish coin collectors for removing coins from circulation. Starting with some 1964 minted coins and continuing through 1967, by decree no mintmarks were allowed, regardless of where a coin was minted. Apparently all three mints were involved in production at irregular times and dates over that 3+year period. Fortunately order was restored in 1968.) I hypothesize that the inconsistencies between mints was the result of different (local and regional) supply sources. Presumaby the cost of shipping (via rail) across country was an unnecessary expense. But why the quality control was so lax is another (as of now) unanswered issue. (Part 3 will address possible differences in coins removed from the ground caused by decades of chemical action. I will also give a brief summary/review of what I consider the 'big picture' results of this study, which at this point seem confusing and muddled, even to me. 😁)
-

VLF Detectors And Depth
GB_Amateur replied to Steve Herschbach's topic in Metal Detector Advice & Comparisons
This doesn't pass the sniff test. Yes, within assumptions what was said is true. IMO you are going way outside of the assumptions that were in place when this statement was made. "The devil is in the details." -

" Treasure " In My Back Yard!
GB_Amateur replied to Joe D.'s topic in Metal Detecting For Coins & Relics
I had a similar experience in a yard where the house was razed and property acquired by the city to be annexed to a local park. (I was first to hunt it. Yeah!!) My suspicion is that children played with their saved pennies (or maybe their parents wanted them out of their hair for a couple hours and said "here, go play with these.") That might be what you're experienceing, too. Now that you've cleaned up the copper, go back and get the silver! -
Welcome, Dasbear! My suggestion is to take a bit of time with your buying decision (I assume the detecting weather isn't great there in the Pacific Northwest). You will find tons of info here on this site, and I would start by familiarizing yourself with site admin Steve Herschbach's database. Not only does that contain overviews (with specs) but also links to other pertinent info. I'm confident you will find a fine performer within your budget. Your choice may still end up being one of the Fisher Gold Bug family members but you may as well view the field before taking the plunge. (How's that for back-to-back cliche's. 😁)
-
Welcome, Dave! All welcome here but particularly glad to have someone with decades of experience. I also look forward to future discussions (and seeing some of your finds).
-
Welcome, stillair1! From your sidebar it looks like you have the tools to recover the goods from near that pier. Hope you also have the weather (but I just read about a storm that hit GB and then Scandanavia, Germany,...). If not, I suspect with the lengthening daylight (N. Hemisphere only 😉) you and we here will soon.
-
Part 1. Flying Eagle and Indian Head Cents (aka FE's and IHP's). 1a. Introduction and experimental setup details. This issue comes up occasionally, and not just for these coins. Periodically coin hunters get spurious/unexpected digital Target ID's (dTID's) for recovered coins and wonder why. There are two obvious explanations: 1) the initial composition (i.e. coin coming from the mint) is anomalous, and 2) the coins composition has somehow been altered since minting. My primary goal in this study was to look into #1, but I also will do a limited peek at #2. It is important to note that in order to investigate #1, no samples should have come from the ground (i.e. results of metal detecting) as it's impossible to distinguish whether or not a spurious dTID is due to #1 or #2 above. An XRF spectrometer (e.g. 'gun') in the right hands would likely be a better investigative tool but at ~$10k that's out of my realm. And since this study also has some relevance in metal detecting for coins, using a modern metal detector makes sense. (More specifically, if the MD response can't be discerned then only numismatists care to know the answer.) I chose to use the currently most popular(?) detector -- Minelab Equinox -- for this study. Here's a photo of my setup: I used settings as close to my standard search setup as possible: 11" coil, Park 1 multifrequency, 5 custom tones, recovery speed = 4, Iron Bias F2 = 0, no notching. Since I was working indoors I had to turn down the gain, but was able to run quietly at 17 which is quite reasonable (including for actual park hunting). I did not want to mess with headphones so listened to the built-in speaker. I built a wooden 'track' structure to restrict the coin's movement to a uniform path and amplitude (left to right excursion distance). Note also the plastic penny holder in the track in the photo which further constrained the coin to a very narrow (within a couple mm of the center) track. This setup allowed a consistent coin height above the coil of 4.5 inches (~11.5 cm). The one thing not carefully controlled was speed of movement (analogous to sweep speed), although I tried different speeds and didn't see a noticeable difference. My typical measurement involved mentally viewing the dTID readout for about 10 side-to-side 'sweeps'. That either led to three values centered on the middle one, or two values centered halfway between them. Thus every recorded datapoint was either an integer (e.g. 23) or half integer (e.g. 22.5). If I had multiple coins of the same date and mintmark, those results are shown in the plots as the average (more accurately stated -- arithmetic mean). When possible I measured at least 3 of each date+mm. These measurements were made over 7 days but I had two standard coins which I checked at every detector turn-on and occasionally during a set of measurements. (I never detected a variation or drift, exhibiting the excellent stability of this detector.) Much of the remainder of this report (including Parts 2 and 3) are plots/graphs of dTID's vs. dates and mintmarks. When comparing different samples, a dTID difference of 0.5 should in general not be interpreted as anything more than experimental measurement variation ('uncertainty'). A difference of 1 has possible meaning and anything larger than 1 is certainly significant. The graphs are particularly good at showing grouped differences as you hopefully see. If there is a missing bar for a particular date, that is either because none were minted or because I didn't have any of those available for this study. 1b. Short history of FE's and IHP's with emphasis on mint specified composition. (Note 1: all numismatic history information here and in future parts is from three sources from Whitman Publishing: 2022 'Redbook' and more detailed books by Snow and Bowers.) (Note 2: only the Philadelphia mint produced cents until 1908.) Large cents were minted from 1793 through 1857. Due in particular to material costs, the transition to small cents was brought about, beginning with a pattern (think 'pre-release production') dated 1856 and coins released for circulation in (and dated) 1857. The first design was known as the 'Flying Eagle' and was short lived, last minted in 1858. (Note, although coins occassionally are minted with a date from the previous year, I will dispense with this distinction from here on.) The FE composition was specified as 88% copper, 12% nickel. These coins have a gray-white color easily distinguished from earlier and later copper alloy cents. (They also are thicker than later 95% copper versions, thus leading to the nickname 'fatty' among detectorists. 😁) In 1859 the design was changed to an American Indian motif obverse, but the composition was kept the same. Note: small cents have been minted every year continuously from 1856 through today (2021). In 1864, a change in composition was made mid-year to 95% copper and 5% tin+zinc. (I have yet to be able to find the breakdown between tin and zinc for IHP's). There were three varieties of 1864 cents -- previous cupro-nickel composition plus the 95% copper without and with the designer's initial 'L'. In the last two years of IHP production (1908 and 1909), the San Francisco mint produced coins with an 'S' under the reverse side wreath. (The plot below does not show those, but in Part 2 I will show a single measurement of a 1908-S.) 1c. dTID measurement results of IHP's. Below is a bar graph showing the dTID results by date for Philadelphia minted FE's, IHP's, and the first seven years of Lincoln Cents, the last of those for comparison. Here are some things worth emphasizing. I had no FE's and only two cupro-nickel (aka 'fatty') IHP examples, both 1863's measuring 16 and 16.5 respectively (with the average of 16.25 plotted). All remainding IHP datapoints are for 95% copper, 5% tin+zinc (tin/zinc ratio unknown). Immediately prior to the minting of Lincolns the tin and zinc breakdowns for those new breed of coins was nailed down at 3% tin and 2% zinc (from Bowers book linked above). However, I don't know what the tolerance specification was. The mint bought sheets of stock from outside suppliers. I'm pretty sure they had lab equipment and technicians to spot check composition of those purchases. However, as you'll see from these posts (especially in part 2), variations are evident outside of the specified breaks or changes in composition that occurred around WWII and later. My conclusion is that with the possible exception of out-of-tolerance composition(?) (e.g. 1864, 1882, 1884), the composition prior to 1894 appears consistent. (Note: based upon what I know at this point, it's certainly possible that the suppliers were given wide allowance to make the breakdown of tin and zinc anywhere from 0% to 5% each! In that case these data could reflect such allowed tolerance variations.) From 1894 thru 1904 the composition looks different from previously and also quite internally consistent. Then there's an apparent change around 1905 for the last 3-5 years of Philadelphia minted IHP's. It gets more interesting for the early (Philadelphia) Lincolns. Recall above I mentioned the composition for Lincolns was specified as 3% tin, 2% zinc (and not changed AFAIK until WWII) yet there is a clear change starting ~1911. What I think is going on is that the mint had surplus sheets left over from the IHP production years and rather than to scrap them they just decided to use up that stock. BTW, I measured six 1910 (plain = Philadelphia) samples, three reading 20 and three reading 23. So apparently the change occurred during the 1910 production year. 20 is low even for the late IHP's. Could some of the 1910's have been minted from stock used in even earlier years? (This has been a very long post. As such I'll stop here and make new posts in this thread for the continuation.)
-
Three technologies are currently on the market: IB/VLF, PI, and ZVT, and I don't know how much difference there is between PI and ZVT. Given that only one ZVT detector is currently sold and it costs an arm and a leg, only its developer (patent owner) is likely to have a say there. Could that technology even be used for practical all-around detecting or is it confined to being a specialty (native gold) niche detector? Maybe Ground Penentrating Radar (GPR) can be included among the technologies -- Nokta/Makro has that on the market for hobbyists (well, cheaper than a ZVT, anway). In the affordable (to most hobbyists) realm, better software signal processing is possible. Also many have requested a VLF/PI hybrid over the years, but AFAIK no developer has done that successfully. (The Geotech forum members likely be able to say more on the feasibility of that.) If such a hybrid comes to market I doubt it will be cheap, either. Still, gold hunters (native gold and jewelry) might still be a lucrative market for it. IMO, improved signal processing seems to be the lowest hanging fruit, but that likely is an incremental improvement, not anything revolutionary.
-
Welcome JN! Silver always pleases me, even if it's the most beat up Roosie or a tiny piece of jewelry. I can't begin to relate to the tragedy you've been through losing your wife, but I'm glad to see you still have a positive attitude towards life. 👍 Get well and hit the ground running (well, as soon as it thaws anyway).
-

15" Nox It Out Of The Park!
GB_Amateur replied to Againstmywill's topic in Metal Detecting For Coins & Relics
-

7 Days Of Arizona Detecting With The 6000
GB_Amateur replied to jasong's topic in Detector Prospector Forum
How much material is actually analyzed with an assay? One of the problems with XRF analysis is that it is a surface measurement, not a bulk measurement. If the sample isn't homogenous then you only get a localized reading which may not represent the entire sample. Is that problem non-existant for an assay? Hope I'm not getting OT here. On the gold mining cable shows they sometimes pan one panful (or two or three), count the colors, and decide if the ground they took it from is worth putting into production. But the more careful miners then run a much larger sample (e.g. 100 cubic yards) through a trommel+sluicebox and get a much more reliable determination of the expected yield before committing the time, labor, fuel cost, etc. to production. I suppose sometimes the pan-only thing is just for 'show', I don't know. -

7 Days Of Arizona Detecting With The 6000
GB_Amateur replied to jasong's topic in Detector Prospector Forum
You have the attitude of a (good) scientist. Wonder why that is.... 😁 Calibration and consistency checking are so important but along with things like statistics, are seldom taught in beginning and intermediate (even many advanced) classes. OK, I'll include a side story which illustrates some of the points you are aware of. On a particular popular current cable TV show (which I won't name to hopefully avoid the usual vindictive attacks) they've been using an XRF gun to determine the presence of precious metals. Of course they are looking for gold and silver, but the spectral analysis shows many more elements. Consistently (well, at least on a small handful of screenshots I've seen) their list shows considerably more Iridium than gold! Yet iridium is not only rarer, but would likely only be found if naturally occurring in their location whereas gold (it is hoped) is from hidden treasure. So what's up? Of course they never mention this anomaly; I don't think it's because they are trying to bury it but rather due to a) they aren't focused on that element, and b) they don't really understand their instrument. (Hopefully it's not because their instrument is improperly calibrated, using improperly written software, etc.) (Now I'm getting a bit technical, but hopefully at least the gist of this is understandable by most readers.) The innermost electron shell of atoms -- the most tightly bound and thus the most energetic -- is called the K-shell. The second innermost shell (less energetic electrons) is the L-shell. In some cases there is a near overlap of high atomic number elements (such as gold, platinum, lead) L-shell electrons with the K-shells of lower atominc number elements such as copper and zinc. Because of the way an XRF spectrometer works, specifically when under a certain calibration, only a part of energy space is viewed. In this (TV show) case it is viewing around the 10 keV energy region which is where those heavy element L-shell emissions lie, and also where some of the lower atomic weight metals' K-shell emissions occur. As a specific (and pertinent here) case, Iridium's L-alpha line energy is 9.175 keV and Copper's K-beta line is 8.905 keV. That's pretty close (<0.3 keV separation) and I hypothesize the instrument is interpreting a copper signal as an Iridium signal, leading to a spurious report. The problem here comes down to whether or not the gold signal is from gold or a low atomic number common metal. It probably does indicate gold, but IMO it requires more than just accepting the simple (and in this case tiny) XRF signal. It appears to be a classic case of confirmation bias. There are ways around this ambiguity, for example in the case of L-lines, requiring presence (and thus a coincidence) of both L-alpha and L-beta lines, which are (in the case of the heavies) sufficiently separated in energy (e.g. 1.5 keV separation for Iridium) to be resolvable. I would hope the instruments use this technique, but it doesn't appear to be the case for the one in use on that TV show. -

7 Days Of Arizona Detecting With The 6000
GB_Amateur replied to jasong's topic in Detector Prospector Forum
An XRF (spectrometer) 'gun' in the hands of someone who both a) knows how to operate it properly and b) knows how to interpret the big picture it helps paint in a geological sense. Throw in c) -- a forum member and contributor -- and you've got the trifecta. There's more to this site than eye candy, but I like that too. -

Received My Bh-01 Bone Conduction Headphones Today
GB_Amateur replied to Steelheader's topic in XP Deus II Forum
I like what Garrett did with their Z-Lynk system. It's incorporated into their higher end detectors but they've made it universal. "Our headphones. We can handle that. Your headphones. We can handle that....") I use their transmitter and receiver set (in concunction with my headphones) with all my detectors except the ML Equinox (where I use the proprietary WM08 receiver). You're good at the garage mods, Joe. So are phrunt and Steve H. and several others here. I wonder if you guys ever even bother unplugging your soldering irons. 😁 But there are reasons companies discourage this -- those people with 10 thumbs, or more likely it's 10 big toes grafted onto their hands -- and then after they've botched things they blame the company. Funny how people who can't do a stitch are also the ones who refuse to take responsibility for their own actions.